Recommendation 6. Invest in biomedical research to create a strong foundation for developing innovative, high-value cancer drugs.
A strong research infrastructure and workforce are essential to develop and deploy innovative, high-value drugs that potentially cure or, if not cure, significantly extend and improve the lives of cancer patients. The U.S. has long been a leader in biomedical research and pharmaceutical innovation, in large part because of cross-sector investment by government, industry, and nonprofit organizations.1 A vibrant discovery ecosystem is essential to ensure that the cancer drug pipeline continues to produce high-value products that benefit all patients.
The National Institutes of Health (NIH)—with an annual budget of nearly $32.3 billion2—is the world’s leading funding organization for biomedical research.3 The basic, translational, clinical, and population sciences research carried out by NIH-supported investigators has helped elucidate the molecular underpinnings of several cancer types and contributed to development of novel therapies—such as imatinib (Gleevec) and ipilimumab (Yervoy)—that have dramatically improved outcomes for patients. In addition to contributing to the development of new drugs, NIH also conducts clinical trials to determine the best ways to use drugs in real-world settings; for example, the National Cancer Institute Molecular Analysis for Therapy Choice Trial (NCI-MATCH)—which is being carried out by collaborators across the country—is testing the effectiveness of several cancer drugs in patients with specific mutations.4 These efforts and others have been driven by the creativity and hard work of numerous researchers, including many who immigrated to the United States. In addition, NIH training grants and career development programs play a critical role in building the U.S. biomedical research workforce.
The NIH budget has not kept pace with inflation since 2003.
NIH historically has enjoyed bipartisan congressional support, most recently demonstrated by passage of the 21st Century Cures Act, which provides NIH with a bolus of additional funding for special initiatives such as the Precision Medicine Initiative and the Cancer Moonshot.5 Pharmaceutical companies also have emphasized the critical role of NIH in funding the types of early-stage research that their companies cannot do.6 However, over the past 15 years, the NIH budget has not kept pace with inflation (Figure 6). Despite budget increases in the past two fiscal years, NIH’s capacity to support research remains far below 2003 levels. The Panel urges the President and Congress to provide sustained, predictable funding for NIH that, at a minimum, keeps pace with inflation. NIH funding is essential to the National Cancer Program and will lay the foundation for development of innovative drugs that provide high value to cancer patients. Failure to invest in NIH will threaten the United States' role as a global leader in the biomedical sciences and future progress against cancer.
Figure 6
NIH Appropriations, Fiscal Years 2003-2017
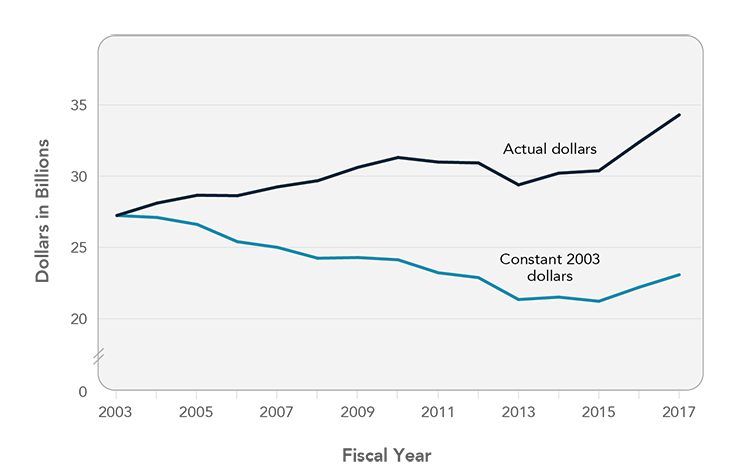
Source: Federation of American Societies for Experimental Biology. NIH research funding trends [Internet]. Bethesda (MD): FASEB; [updated 2017 Jun 26; cited 2017 Oct 6]. Available from: http://faseb.org/Science-Policy-and-Advocacy/Federal-Funding-Data/NIH-Research-Funding-Trends.aspx
The Panel also urges continued commitment to cancer research by other sectors, including nonprofit organizations, venture capital companies, and the biopharmaceutical industry. Sustained investment from multiple sectors is needed to build and maintain a pipeline of oncology drugs that provide transformative rather than incremental benefits. Biopharmaceutical companies play a particularly critical role in conducting clinical trials necessary to determine the safety and efficacy of new drugs and drug combinations. U.S. laws, regulations, and policies should encourage investments in cancer research and drug development.
As noted in recent reports from the Panel7 and the Cancer Moonshot Task Force,8 a culture of collaboration is essential for catalyzing new scientific breakthroughs. There are many opportunities for stakeholders to work together, including:
- Increasing availability of drugs for preclinical research to gain insights into mechanisms of action and potential biomarkers;
- Sharing data, including clinical trial outcomes, to inform future research;
- Collaborating to test promising combination therapies, including combinations of drugs manufactured by different companies; and
- Engaging patients and patient advocates to ensure that research is aligned with patients’ needs and priorities.
Though cross-sectional collaborations and partnerships can be challenging, researchers, research-funding organizations, biopharmaceutical companies, and patients should find ways to work together to accelerate development of innovative new cancer drugs that will extend and improve patients’ lives. Some efforts are under way to facilitate these types of collaboration. One example is the National Cancer Institute agent formulary (NCI Formulary),9 a public-private partnership between NCI and biopharmaceutical companies that provides NCI-designated Cancer Center investigators rapid access to agents for cancer clinical trial use or preclinical research. Additional initiatives and platforms that facilitate collaboration should be established and supported.
References
- Dorsey ER, de Roulet J, Thompson JP, Reminick JI, Thai A, White-Stellato Z, et al. Funding of U.S. biomedical research, 2003-2008. JAMA. 2010;303(2):137-43. Available from: https://www.ncbi.nlm.nih.gov/pubmed/20068207
- National Institutes of Health. Budget [Internet]. Bethesda (MD): NIH; [updated 2017 Mar 6; cited 2017 Apr 19]. Available from: https://www.nih.gov/about-nih/what-we-do/budget
- Viergever RF, Hendriks TC. The 10 largest public and philanthropic funders of health research in the world: what they fund and how they distribute their funds. Health Res Policy Syst. 2016;14:12. Available from: https://www.ncbi.nlm.nih.gov/pubmed/26892771
- National Cancer Institute. NCI-MATCH Trial (Molecular Analysis for Therapy Choice) [Internet]. Bethesda (MD): NCI; [updated 2017 Jun 6; cited 2017 Dec 22]. Available from: https://www.cancer.gov/about-cancer/treatment/clinical-trials/nci-supported/nci-match
- Hudson KL, Collins FS. The 21st Century Cures Act—a view from the NIH. N Engl J Med. 2017;376(2):111-3. Available from: https://www.ncbi.nlm.nih.gov/pubmed/27959585
- Basken P. Called to the White House, business leaders attest to NIH's value. The Chronicle of Higher Education [Internet]. 2017 May 9 [cited 2017 May 18]. Available from: http://www.chronicle.com/article/Called-to-the-White-House/240031
- President's Cancer Panel. Improving cancer-related outcomes with connected health: a report to the President of the United States from the President's Cancer Panel. Bethesda (MD): the Panel; 2016 Nov. Available from: https://prescancerpanel.cancer.gov/report/connectedhealth
- Cancer Moonshot Task Force. Report of the Cancer Moonshot Task Force. Washington (DC): the White House; 2016 Oct 17. Available from: https://medium.com/cancer-moonshot/report-of-the-cancer-moonshot-task-force-executive-summary-e711f1845ec
- National Cancer Institute. NCI Formulary [Internet]. Bethesda (MD): NCI; [cited 2017 Apr 17]. Available from: https://nciformulary.cancer.gov
